Change of State
Change of State: Overview
This Topic covers sub-topics such as Condensation, Calorimeter, Solidification, Principle of Calorimetry, Change of State, Specific Latent Heat, Specific Latent Heat of Fusion and, Specific Latent Heat of Vapourization
Important Questions on Change of State
An unknown solid of mass of solid at is allowed to sink in of water at . Calculate the specific heat of the unknown solid in the units of if the final temperature of water becomes . Specific heat of water is and density of water is .
Give explanation of Melting by Molecular Model.
water at is mixed with another liquid of mass at . After mixing, they attain a mutual temperature of . If the specific heat capacity of water is , determine the specific heat capacity of the other liquid in the same unit.
Identify the melting point of ice from the graph.
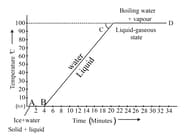
Define specific latent heat by vaporization.
Describe the principle of method of mixtures.
State the principle of method of mixtures.
Caloric theory of heat was abandoned by Joule's experiment.
What does the principle of calorimetry state?
Conversion of liquid into vapours is called _____.
Condensation takes place when water becomes ice.
The transformation from one state of matter to another is known as change of state.
Change of water into ice, on freezing.
Hint: This state of matter has definite shape and volume.
